Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir
Abstract
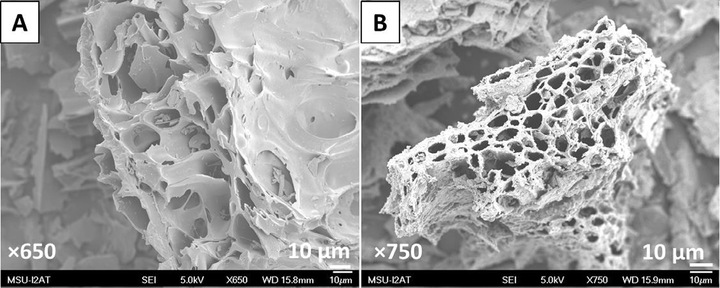
Magnetic biochar (MBC) was produced by magnetite (Fe3O4) precipitation onto Douglas fir biochar (NBC) that had been generated by wet fast pyrolysis. Treating an aqueous Fe3+/Fe2+ solution with NaOH induced Fe3O4 to nucleate and deposit on NBC. The NBC and the resulting MBC were used to remove Pb2+ and Cd2+ from water. Both biochars were characterized by SEM, EDX, TEM, PZC, XRD, elemental analysis, and surface area measurements. Batch sorption studies were carried out from pH 2-–7 with adsorbate concentrations from 10 to 250mg/L at 25, 35 and 45 °C. MBC and NBC suspensions in the contaminated solutions were vortexed for two min. Then MBC was magnetically removed, while NBC required filtration. Remediated solutions were then analyzed using AAS. The amounts of lead and cadmium adsorbed onto both NBC and MBC were lower at low pH values and increased with increasing pH. The Langmuir and Freundlich adsorption isotherm models were applied to describe equilibrium data. The maximum Langmuir adsorption capacities at pH 5 and 45 °C for Pb2+ and Cd2+ uptake were ∼40 and ∼16mg/g for NBC and ∼27 and ∼11mg/g for MBC, respectively. NBC and MBC recycling was carried out after metal ion extraction with 0.1 M HCl and water. Adsorption kinetics of NBC and MBC were compared with four other biochars (mixed feed, magnetized mixed feed, pinewood and magnetized switchgrass). Adsorption equilibria of Pb2+ and Cd2+ onto both NBC and MBC were reached within 2 min while the other biochars required from 2 to 20 h. NBC and MBC have potential as low cost, green adsorbents for rapid lead and cadmium remediation as replacements for more expensive commercial activated carbon.
Supporting information can be found here.