Planar, Stair-Stepped, and Twisted: Modulating Structure and Photophysics in Pyrene- and Benzene-Fused N‐Heterocyclic Boranes
Abstract
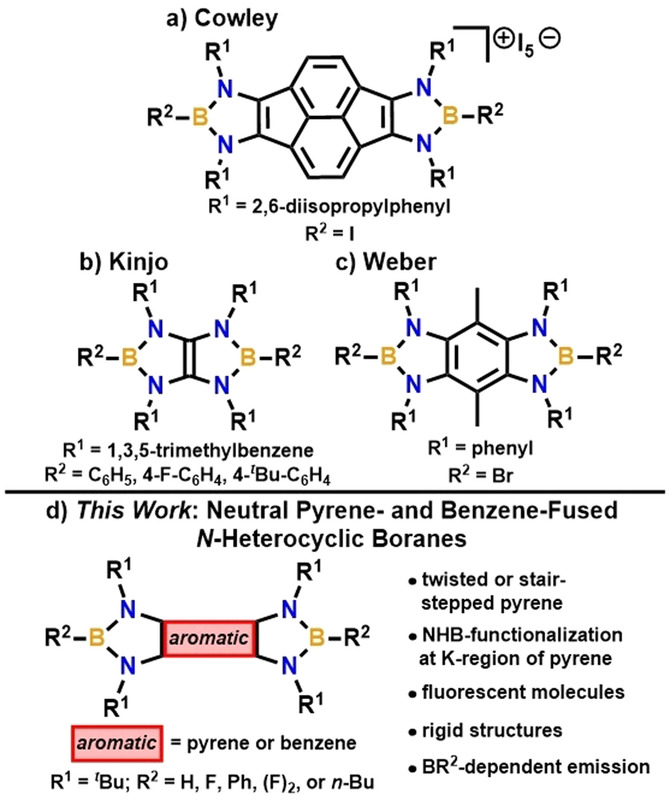
Because of their rigidity, polycyclic aromatic hydrocarbons (PAHs) have become a significant building block in molecular materials chemistry. Fusion or doping of boron into PAHs is known to improve the optoelectronic properties by reducing the LUMO energy level. Herein, we report a comprehensive study on the syntheses, structures, and photophysical properties of a new class of fused N–heterocyclic boranes (NHBs), pyrene‐ and benzene‐linked in a “Janus‐type” fashion (2–4, 6–9, and 11). Reemarkably, these examples of fused NHBs display fluorescent properties, and collectively their emission spans the visible spectrum. The pyrene‐fused NHBs all display blue fluorescence, as the excitations are dominated by the pyrene core. In notable contrast, the emission properties of the benzene‐fused analogues are highly tunable and are dependent on the electronics of the NHB fragments (i.e., the functional group directly bound to the boron atoms). Pyrene‐fused 2–4 and 11 represent the only molecules in which the K‐region of pyrene is functionalized with NHB units, and while they exhibit distorted (twisted or stair‐stepped) pyrene cores, benzene‐fused 6–9 are planar. The electronic structure and optical properties of these materials were probed by computational studies, including an evaluation of aromaticity, electronic transitions, and molecular orbitals.
Supporting information can be found here.