Sugar Dehydration
Convert sugar into carbon with sulfuric acid
Summary
Turn sugar into carbon by dehydrating sugar with strong sulfuric acid.
Materials
- 100 mL of 16 M sulfuric acid
- 85 g of granulated sugar
- Stir rod
- Beaker
- Hot gloves
Procedure
Perform demonstration in a well ventilated area.
- Add sulfuric acid to a beaker
- Add sugar to a beaker
- Stir the mixture completely
- Wait 2–3 minutes for reaction to complete
- Apparatus will be HOT. Do NOT touch until cool.
Discussion
The sugar dehydration experiment (also known as the carbon snake experiment) is based on the ability of concentrated sulfuric acid to dehydrate sugar via the following process:
$$\mathrm{C_{12}H_{22}O_{11}}(g) \longrightarrow 12\mathrm{C}(s, \mathrm{~graphite}) + 11\mathrm{H_2O}(l)$$
We see sugar is converted to solid, black graphite and water is a product (hence dehydration).
When table sugar and concentrated sulfuric acid are combined, an exothermic reaction occurs that produces vaporized water and carbon dioxide. The vaporized water and carbon dioxide are responsible for the expansion of the mixture inside the container. As water and carbon dioxide are produced, the mixture becomes inflated and expands beyond the opening of its container, creating a snake-like appearance. The overall chemical equation can be seen below.
$$\mathrm{C_{12}H_{22}O_{11}}(g) + \mathrm{H_2SO_4}(aq) + \dfrac{1}{2}\mathrm{O_2}(g) \longrightarrow 11\mathrm{C}(s) + \mathrm{CO_2}(g) + 12\mathrm{H_2O}(g) + \mathrm{SO_2}(g) + \mathrm{heat}$$
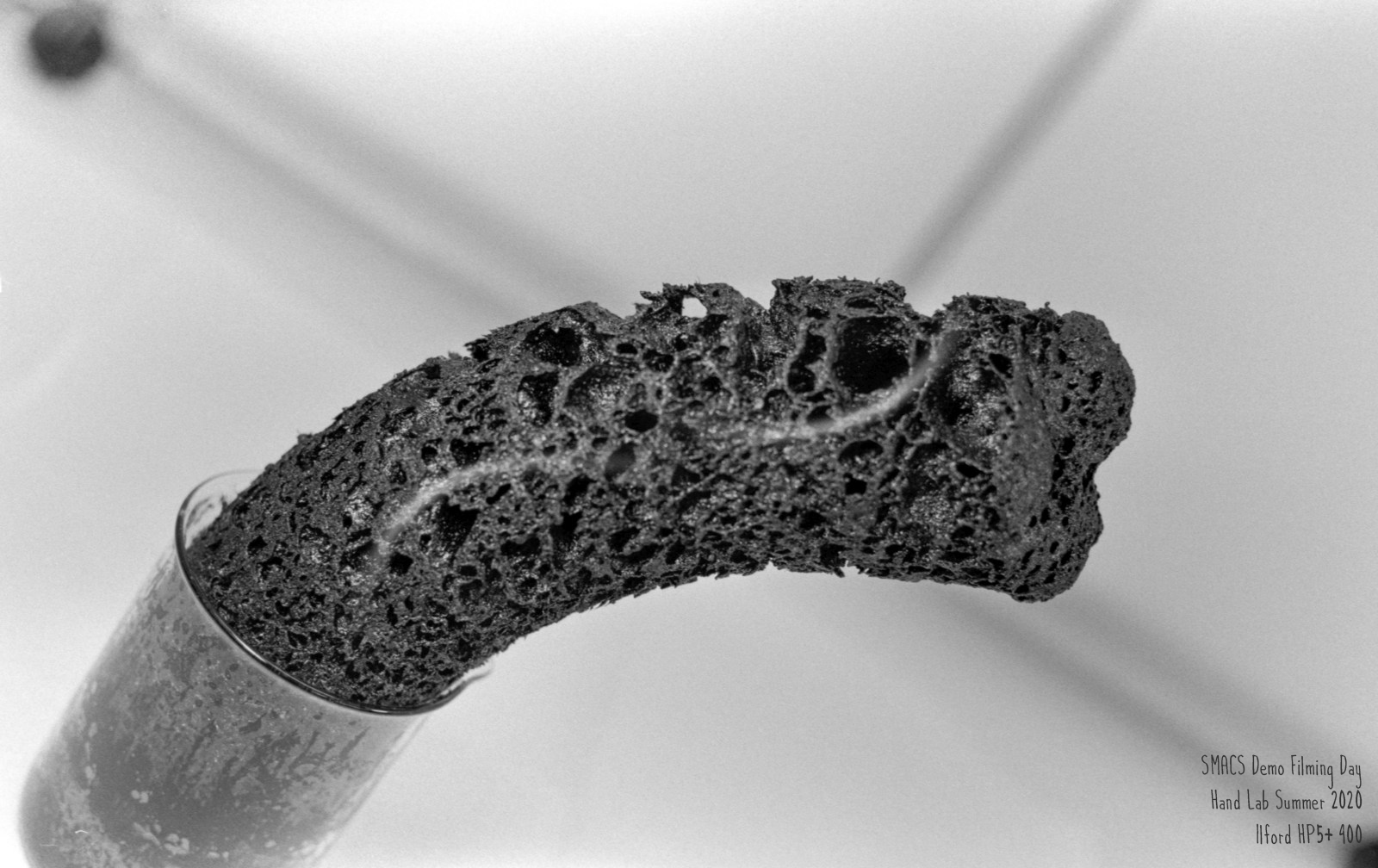
If you look closely, you can see steam coming off the column of graphite!
